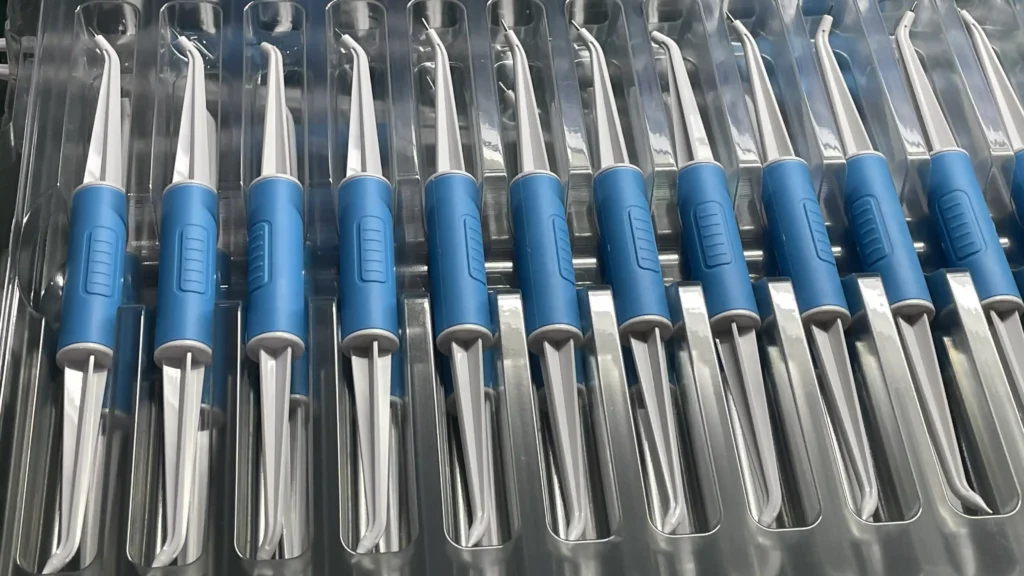
As a reliable injection molding manufacturer, YG focuses on custom medical injection molding and has passed ISO 13485 certification. We understand the unique obstacles that buyers of medical components in the EU and the US face when sourcing china plastic injection molding medical parts.
Recently, we collaborated with a global oral care brand to provide custom components for their new equipment, leveraging medical device injection molding expertise and overmolding technology (PP base + TPU outer layer).
In this case study, we will break down how we meet the strict medical injection molding requirements and turn them into long-term customers. Let’s get started!
1. Project Overview: Meeting the Client’s medical injection molding requirements
Table of Contents
ToggleOur client is a Western European medical equipment manufacturer specializing in oral health care. They came to us with a clear goal: to find a reliable partner to customize medical injection molding and create a durable and biocompatible component for their dental equipment. Specifically, they require plastic injection molding medical parts that can withstand repeated oral use while providing anti-slip grips, all of which comply with EU/US standards.
Key Requirements & Challenges
- Regulatory Alignment: This component must comply with the FDA and CE medical plastic injection molding rules, which are a necessary condition for entering the global market.
- Material Bonding: Their design requires a rigid PP base and a soft TPU outer layer, which is a common pain point for injection molding medical plastics (PP is naturally non-adhesive, making a strong bond tricky).
- Precision: Like all the best plastic injection molding medical parts, this component requires strict dimensional tolerances to seamlessly fit with other device components.
If you are sourcing injection molding medical parts, you know that these challenges may delay the product’s release. We make solving them our top priority.
2. The Solution: Custom Medical Injection Molding and Cleanroom Expertise
To meet the requirements of our client, we have combined meticulous material selection, optimized medical injection mold Settings, and cleanroom medical injection molding technology – all of which are at the core of our medical injection molding services. This is how we do it:
Step 1: Sourcing Medical-Grade Materials
Not all plastics work for medical use. We selected:
- Base Material (PP): Medical-grade PP material that meets FDA certification. Due to its biocompatibility and resistance to oral care chemicals, it is an ideal choice for injection molding medical plastics.
- Overmold Material (TPU): Medical-grade TPU, with Shore A hardness, strikes a balance between comfort and durability, and has passed the cytotoxicity test, which is crucial for medical device injection molding that comes into contact with oral tissues.
Step 2: Refining the Medical Injection Molding Process
Overmolding two dissimilar materials requires precision—especially for medical injection molding. We divide this process into two controlled stages:
- First Shot (PP Base): We use a closed-loop machine to optimize temperature and pressure to prevent bubbles that might weaken injection molding medical parts or capture bacteria.

- Second Shot (TPU Overmold): Before injecting TPU, we treat the surface of the PP to enhance adhesion (many medical injection molding services overlook this step). We also adjusted the temperature and speed of the medical injection mold to ensure uniform TPU coverage – no thin spots or excess material.
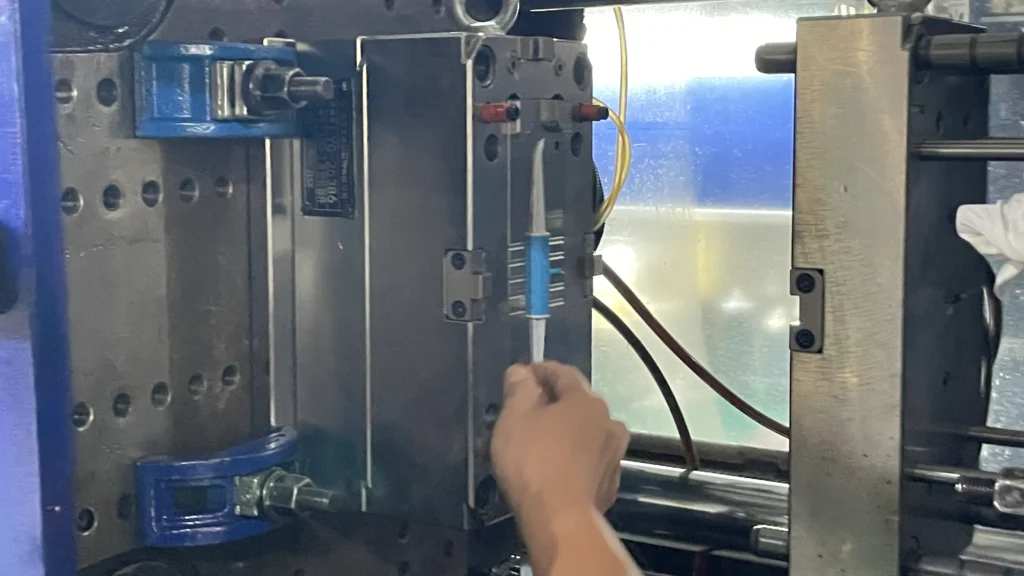
Step 3: Cleanroom Medical Injection Molding for Sterility
All medical devices take place in our Class 8 (100,000) cleanroom, which meets the requirements of medical ISO 13485 injection molding. This minimizes dust, microorganisms, and contaminants to the greatest extent, ensuring that the ingredient meets the sterility standards for oral use by customers.
3. Execution & Quality Control: Medical ISO 13485 Injection Molding in Action
For injection molding medical parts, “good enough” is not enough. Our medical ISO 13485 injection molding system guides every step – from raw material import to delivery – to ensure consistency and traceability.
How We Ensured Quality for the Best Plastic Injection Molding Medical Parts
- Incoming Material Checks: We conduct 100% testing on the biocompatibility of PP and TPU batches and reject any batches that do not meet the requirements of medical injection molding.
- In-Process Testing: During the production process, we use high-precision three-coordinate measuring machines to regularly inspect the dimensional accuracy of injection molding medical parts. We also conducted a peel test to confirm the bonding strength of PP/TPU.
- Full Traceability: Each component has a unique serial number, which is linked to the production log, which is crucial for the review of medical device injection molding.
Have you ever worked with a medical injection molding company that couldn’t track the history of its parts? This is a huge risk that we have eliminated for medical equipment.

4. Results: Long-Term OEM Medical Injection Molding Partnership
The result is self-evident – proving our expertise in custom medical injection molding:
- 100% Regulatory Compliance: This component has passed FDA and CE tests for the first time – no rework, no delay, and meets the requirements for medical plastic injection molding.
- 99.2% First-Pass Yield: Compared to our previous suppliers, we have reduced waste and cut costs by 8%, marking a significant achievement in purchasing China plastic injection molding medical parts.
- On-Time Delivery: We met their required delivery time, which was crucial for the successful launch of their product.
The purchasing manager of the client said this:
“Working with YG has changed the game. As a supplier providing OEM medical injection molding services, they do not merely send plastic parts. They understand our medical injection molding requirements and solve our bonding problems. We have reached a consensus on long-term cooperation.”
5. Choose YG as Your Medical Device Injection Molding Supplier
If you are looking for a supplier of injection molding medical components in China, you need a reliable partner – FDA, CE, ISO 13485. Here are what sets us apart:
- Comprehensive services: We offer end-to-end medical injection molding services, from medical injection mold design to cleanroom medical injection molding and post-production testing.
- OEM/ODM Expertise: Our OEM medical injection molding capabilities enable us to customize solutions based on your exact equipment requirements.
Are you ready to skip the headache of an inexperienced medical plastic injection molding producer? Let’s talk about how to support your next project. Contact us now→